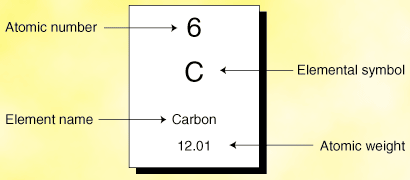
The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. If you're seeing this message, it means we're having trouble loading external resources on our website. The atomic number of each element increases by one, reading from left to right. Block Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Atomic Number: 8 Atomic Mass: 15.9994 amu Melting Point:-218.4 °C (54.750008 K, -361.12 °F) Boiling Point:-183.0 °C (90.15 K, -297.4 °F) Number of Protons. The sum of the atomic number Z and the number of neutrons N gives the mass number A of an atom. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity.
An element's atomic number tells us the number of what?
1 Answer
Explanation:
So if
And if
And if
...........................
And if
And the next concept to grasp is that of

If
The atomic mass printed on the Periodic Table is the weighted average of the isotopes. And as
Related questions
S Block Atomic Number
Moseley's Discovery - The Modern Concept of Atomic Number
Today, we know that the atomic number gives the number of protons (positive charges) in the nucleus. This was the discovery made by Henry Gwyn-Jefferies Moseley. He found that certain lines in the X-ray spectrum of each element moved the same amount each time you increased the atomic number by one.
Rutherford (in 1914) described Moseley's discovery thus:
'Recently Moseley has supplied very valuable evidence that this rule [atomic numbers changing by one from element to element] also holds for a number of the lighter elements. By examination of the wave-length of the characteristic X rays emitted by twelve elements varying in atomic weight between calcium (40) and zinc (65.4), he has shown that the variation of wave-length can be simply explained by supposing that the charge on the nucleus increases from element to element by exactly one unit. This holds true for cobalt and nickel, although it has long been known that they occupy an anomalous relative position in the periodic classification of the elements according to atomic weights.'
I. Atomic Structure: 1903 - 1911
Exactly where the positive protons (and the negative electrons) were in the atom took time to be worked out. Keep in mind that the electron (the first sub-atomic particle discovered) was not discovered until 1897.
(1) J.J. Thomson in 1903, had electrons as negative particles with mass, while the positive charge was spread out through the space of the atom.
(2) In 1911 Rutherford announced his atomic model: (a) a nucleus - a dense concentration of positive charge with (b) electrons orbiting the nucleus in an unspecified manner.
(3) In 1913, Bohr took up the question of where the negative electrons are (in the atom) and Moseley studied where the positive charges were.
By the way, Moseley was part of Rutherford's research group -- having arrived in Manchester just weeks before Rutherford published his great nucleus paper -- when he started his atomic number work. Rutherford was not all that excited by Moseley wanting to study X-rays, but the energy and enthusiasm of the younger man soon wore Rutherford down.
[You might notice that neutrons have not been mentioned. It would not be until 1920 that Rutherford proposed the existence of a neutral particle -- the neutron. Another of Rutherford's students -- James Chadwick -- won the 1935 Nobel Prize for discovering the neutron in 1932.]
Within a few months of Rutherford's nucleus paper being published, the true, physical meaning of 'atomic number' was suggested by A. van den Broek. In 1913, he wrote:
'In a previous letter to NATURE (July 20, 1911, p. 78) the hypothesis was proposed that the atomic weight being equal to about twice the intra-atomic charge, 'to each possible intra-atomic charge corresponds a possible element,' or that (Physik. Zeitschr, xiv., 1912, p. 39), 'if all elements be arranged in order of increasing atomic weights, the number of each element in that series must be equal to its intra-atomic charge.' '
II. Moseley's X-Ray Spectra Work
Moseley's problem was to find a linear relationship between the atomic number and a measureable property of the nucleus. The atomic number increased by steps of one (18, 19, 20, 21, and so on). Moseley needed some function of a nuclear property that increased in the same pattern, that is, by one for each element in turn. He found it in the K line of the X-ray spectra of each element. It turns out that the square root of the frequency moves by a constant value (let's call it 'one unit') for each one unit move by the atomic number.
Why did he choose to study this area for what he needed? We can find the answer in the work of Charles Barkla. He had demonstrated that the elements emitted characteristic X-rays, called K and L rays. These X-rays were independent of the physical or chemical state the element was in. Someone, perhaps Barkla or Bohr or Moseley, realized that this meant the X-rays were characteristic of the nucleus.
So Moseley set about to determine the wavelengths of the K radiation using recently discovered techniques by the father-and-son team of W.L Bragg and W.H. Bragg. It seems to me as I write this that Moseley was pretty confident going into this experiment that all he needed to do was find the proper linear relationship. Getting the equipment working so as to give reliable data was probably the most time-consuming task of the entire research he carried out.

However, the research was carried out and Moseley determined the relationship mentioned above. It was linear, with the frequency square root value moving up the same amount for each one unit jump in the atomic number. Here, using Moseley's data is graph which shows linear behavior:
Gold's Atomic Number
About this data, Moseley himself said:
What Is Lithium's Atomic Number
'We have here a proof that there is in the atom a fundamental quantity, which increases by regular steps as we pass from one element to the next. This quantity can only be the change on the central positive nucleus, of the existence of which we already have definite proof.'
